Publications
Book chapter: 2.2 Synthesis of Monofluoromethylated Compounds
Das, A.; Ramkumar, N.; Veliks, J. Modern Strategies in Organofluorine Chemistry 2; Georg Thieme Verlag KG: Stuttgart, 2026; Vol. 2025/3. DOI: 10.1055/sos-SD-244-00320
Synthesis of Amino Acids With Five-Membered N-Heterocycles in Side Chain
Monofluorinated C1 Synthon Strategy for the Construction of Fluoromethylene‐Linked Bicyclo[1.1.1]Pentane Derivatives
Monofluoromethyl Radical Mediated Halogen-Atom Transfer
Acridinium Photo-Catalyzed Monofluoromethyl Radical Cascade Reaction of Alkenes with Iodine(III) Reagent: A Mechanistic Study
Disassembly of the Escherichia Coli AcrABZ-TolC Efflux Pump by Ligand-mediated Disruption of TolC-AcrA Interfacial Contacts
Metal-Catalyzed Fluoroacetyl Carbene Transfer from Sulfonium Salts
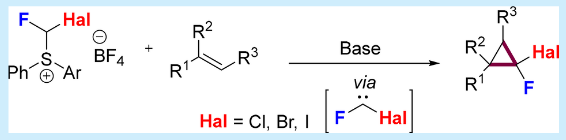
Fluorohalomethylsulfonium Salts as a Fluorohalocarbene Source
Recent Advances in Monofluorinated Carbenes, Carbenoids, Ylides, and Related Species
Sperga, A.; Veliks, J. Chem. Eur. J. 2023, 29, e202301851. DOI: 10.1002/chem.202301851
Photoredox-Catalyzed Direct C-H Monofluoromethylation of Heteroarenes
Ramkumar, N.; Plantus, K.; Ozola, M.; Mishnev, A.; Nikolajeva, V.; Senkovs, M.; Ošeka, M.; Veliks, J. New J. Chem. 2023, 47, 20642-20652. DOI: 10.1039/D3NJ04313D
Visible‐Light Photoredox‐catalyzed Radical Fluoromethoxylation of Olefins
Ramkumar, N.; Sperga, A.; Belyakov, S.; Mishnev, A.; Zacs, D.; Veliks, J. Adv. Synth. Catal. 2023, 365, 1405-1412. DOI: 10.1002/adsc.202300130
Synthetic Access to Fluorocyclopropylidenes
Melngaile, R.; Videja, M.; Kuka, J.; Kinens, A.; Zacs, D.; Veliks, J. Org. Lett. 2023, 13, 2280–2284. DOI:10.1021/acs.orglett.3c00579
Merging Copper(I) Photoredox Catalysis and Iodine(III) Chemistry for the Oxy-monofluoromethylation of Alkenes
Ramkumar, N.; Baumane, L.; Zacs, D.; Veliks, J. Angew. Chem. Int. Ed. 2023, 62, e202219027. DOI:10.1002/anie.202219027
Iron-Catalyzed Fluoromethylene Transfer from a Sulfonium Reagent
Sperga, A.; Zacs, D.; Veliks, J. Org. Lett. 2022, 24, 4474–4478. DOI:10.1021/acs.orglett.2c01757
Synthetic Applications of Monofluoromethylsulfonium Salts
Melngaile, R.; Veliks, J. Synthesis 2021, 53, 4549-4558. DOI:10.1055/a-1548-8240

Sulfonium, (Fluoromethyl)phenyl(2,3,4,5-tetramethylphenyl)-, Tetrafluoroborate(1-) (1:1)
Melngaile, R.; Veliks, J. G. Encycl. Reagents Org. Synth., 2021, pp 1-4. DOI:10.1002/047084289X.rn02379

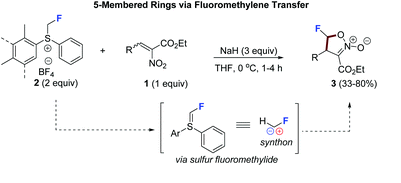
Monofluorinated 5-membered rings via fluoromethylene transfer: synthesis of monofluorinated isoxazoline N-oxides
Sperga, A.; Kazia, A.; Veliks, J. Org. Biomol. Chem., 2021, 19, 2688-2691. DOI:10.1039/D1OB00270H
Residual Solvent Signal of CDCl3 as a qNMR Internal Standard for Application in Organic Chemistry Laboratory
Muhamadejev, R.; Melngaile, R.; Paegle, P.; Zibarte, I.; Petrova, M.; Jaudzems, K.; Veliks, J. J. Org. Chem., 2021, 86, 3890-3896. DOI:10.1021/acs.joc.0c02744
Optimized Monofluoromethylsulfonium Reagents for Fluoromethylene-Transfer Chemistry
Sperga, A.; Melngaile, R.; Kazia, A.; Belyakov, S.; Veliks, J. J. Org. Chem., 2021, 86, 3196–3212. DOI:10.1021/acs.joc.0c02561
trans-Fluorine Effect in Cyclopropane: Diastereoselective Synthesis of Fluorocyclopropyl Cabozantinib Analogs
Veliks, J.; Videja, M., Kinens, A.; Bobrovs, R.; Priede, M., Kuka, J. ACS Med. Chem. Lett., 2020, 11(11), 2146-2150. DOI:10.1021/acsmedchemlett.0c00220
Johnson–Corey–Chaykovsky Fluorocyclopropanation of Double Activated Alkenes: Scope and Limitations
Kazia, A.; Melngaile, R.; Mishnev, A.; Veliks, J. Org. Biomol. Chem. 2020, 18, 1384-1388. DOI:10.1039/C9OB02712B
Diastereoselective Monofluorocyclopropanation Using Fluoromethylsulfonium Salts
Melngaile, R.; Sperga, A.; Baldridge, K. K.; Veliks, J. Org. Lett. 2019, 21,7174-7178. DOI:10.1021/acs.orglett.9b02867

Fluoromethylene Transfer from Diarylfluoromethylsulfonium Salts: Synthesis of Fluorinated Epoxides
Veliks, J.; Kazia, A. Chem. Eur. J., 2019, 25, 3786 –3789. DOI:10.1002/chem.201900349

Enantioselective Rhodium-Catalysed Insertion of Trifluorodiazoethanes into Tin Hydrides
Hyde, S.; Veliks, J.; Ascough, D.M.; Szpera, R.; Paton, R.S.; Gouverneur, V. Tetrahedron, 2019, 75, 17-25. DOI:10.1016/j.tet.2018.11.022

Copper-Catalyzed Insertion into Heteroatom-Hydrogen Bonds with Trifluorodiazoalkanes
Hyde, S.; Veliks, J.; Liégault, B.; Grassi, D.; Taillefer, M.; Gouverneur, V. Angew. Chem. Int. Ed., 2016, 55, 3785-3789. DOI:10.1002/anie.201511954
Towards the Molecular Borromean Link with Three Unequal Rings: Double-Threaded Ruthenium(II) Ring-in-Ring Complexes
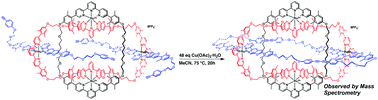
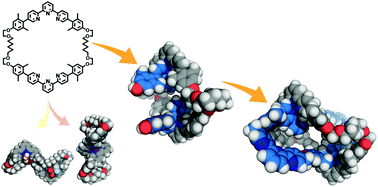
Veliks, J.; Seifert, H.M.; Frantz, D.K.; Klosterman, J.K.; Tseng, J.-C.; Linden, A.; Siegel, J.S. Org. Chem. Front., 2016, 3, 667–672. DOI:10.1039/c6qo00025h
Conformations of Large Macrocycles and Ring-in-Ring Complexes
Klosterman, J.K.; Veliks, J.; Frantz, D.K.; Yasui, Y.; Loepfe, M.; Zysman-Colman, E.; Linden, A.; Siegel, J.S. Org. Chem. Front., 2016, 3, 661-666. DOI:10.1039/c6qo00024j
Three Steps in One Pot: Synthesis of Linear Bilateral Extended 2,2′:6′,2″-Terpyridineruthenium(II) Complexes
Veliks, J.; Blacque, O.; Siegel, J.S. Inorg. Chem., 2014, 53, 12122-12126. DOI:10.1021/ic501946f

Linear Bilateral Extended 2,2′:6′,2′′-Terpyridine Ligands, Their Coordination Complexes and Heterometallic Supramolecular Networks
Veliks, J.; Tseng, J.-C.; Arias, K.I.; Weisshar, F.; Linden, A.; Siegel, J.S. Chem. Sci., 2014, 5, 4317–4327. DOI:10.1039/c4sc01025f